Measles
Kid’s Stuff
Koplik’s spots sighted.
Super spreader Morbilli
Has become entrenched.
“Full many a gem of purest ray serene
The dark, unfathom’d caves of ocean bear:
Full many a flower is born to blush unseen,
And waste its sweetness on the desert air.”
-From “Elegy Written in a Country Church-Yard”
By Thomas Grey
Full many brilliant, resourceful scientists labor unceasingly in the quest to better the plight of humanity. Alas, only a few are acknowledged and remembered by history. Many are familiar with the names of Louis Pasteur, Edward Jennings, Alexander Fleming, and Jonas Salk, even if it was just to remember them for a social studies quiz. But many others have labored tirelessly and unyieldingly to help humanity rid itself of horrible diseases and suffering. Put the name of a Persian from the 9th and 10th centuries, Abu Bakr Muhammad ibn Zakariya al-Razi (known in the West as Rhazes), and a Dane, Peter Panum, who lived a thousand years later, on your social studies test, and it’s a good bet that few would get the correct answer. But they both contributed greatly to the world’s understanding and approach to medicine and disease, enabling future generations to develop treatments that benefit us today. Worlds and centuries apart, these two remarkable physicians illuminated facts about a disease that has infected humans mercilessly for at least a thousand years, rubeola. Most of us recognize it by its common name, measles. Their work was invaluable for that disease and trendsetting in the foundation of medical practice.
Rhazes was a true master. He was born in 865 AD in the foothills near present-day Tehran and spent most of his life there. At an early age, he studied music but later became interested in alchemy, philosophy, and medicine. Because of his gifts for learning and teaching, his reputation spread throughout the country, and he became famous in several fields, especially for his knowledge of medicine. Rhazes was a prolific writer, and his works were copied on a large scale and distributed widely. Since many were translated into Latin and Greek, he was considered his era's leading researcher and instructor.
Rhazes wrote over 200 volumes, a phenomenal number for his time, but his greatest influence came from his medical books. In them, he described a philosophy unknown at the time, the use of scientific logic to reach conclusions. He was reportedly the first physician to separate patient groups into two sets, one receiving a treatment, the other not, then evaluating the technique's usefulness. This is so commonly done today that none questions it, but it was unheard of in the ninth and tenth centuries. He wrote a book called “For One Who Is Not Attended by a Physician or a Medical Advisor,” a volume for the poor and ordinary citizens, the first of its kind. One of his greatest influences was distinguishing smallpox from measles, which up until his time, had been considered varied forms of the same disease. Though Middle Eastern, Rhazes’ influence over Western medicine for the next thousand years was profound, not only for presenting medical facts and successful treatments but in the scientific approach to what was before mainly in the spiritual and superstitious domain.
The study of infectious diseases (or most diseases, for that matter) isn’t conducted in a tightly controlled laboratory setting but in the real world with all its confusing extraneous, and sometimes complicated, factors. But in 1846, the embryonic period of the science of infectious diseases, an ideal situation for studying an infectious disease presented itself. It was an outbreak of measles on the Faroe Islands, located midway between Norway and Iceland. The Faroes were uniquely sequestered: the locals stayed confined to their island habitat, and strangers rarely ventured in. It was isolated for many years from outside influence, except for the occasional seaman or traveler passing through. When measles struck the island, the population was naïve to the virus; all inhabitants were susceptible. It provided the perfect opportunity for study. The Danish Ministry of Health dispatched Dr. Peter Panum to the islands to evaluate the measles epidemic and report his findings. Dr. Panum and his team studied the natural history of the disease, including incubation time, duration of illness, and lifelong immunity. Dr. Panum undertook his task with alacrity and made many astute observations about the disease, which he published in a treatise titled “Observations Made During the Epidemic of Measles on the Faroe Islands in the Year 1846.” The work was extremely important in describing the disease, especially its spread, when very little was known about the subject. It laid the groundwork for others to make similar observations about other diseases. John Snow is generally considered the father of epidemiology, but Panum’s work preceded his by eight years.
Even though the people and physicians of olden times didn’t understand the microbial cause of infectious diseases, they recognized, in a general way, their transmission from the infected person to the non-infected. The term “social distancing” was not used, of course. Still, through word of mouth, medical observance, and in some cases, governmental laws (as in the case of quarantine), most people knew to avoid those ill and showing overt signs of an illness. Measles was such a disease. The problem, of course, is that the disease can be spread before the hallmark signs are noticeable. Workers like Rhazes and Peter Panum laid the groundwork for a scientific approach to the field of epidemiology and the betterment of public health.
Humans are the only creatures that can contract measles, but other animals have diseases caused by closely related viruses. Most dog owners know they must get their pet shots for the disease distemper, even though few actually know what distemper is. It’s a disease similar to measles, at least the virus causing it is. When exposed to the distemper virus, most dogs become very ill, and some die. Sickened dogs run a very high fever, have runny noses, and develop profound diarrhea. They are listless with a reduced appetite. There is no cure for distemper, merely palliative care. Domestic dogs aren’t the only members of the canine family that are vulnerable to the virus: wolves, foxes, and coyotes all can show the same symptoms. The range of animals susceptible to measles-related viruses doesn’t stop with canines. Skunks, raccoons, large wild cats, and sea mammals such as dolphins have their own strains of a closely related virus that can make them seriously ill.
A cattle disease caused by a virus of the same type is of particular interest to humans. Although unknown to today’s layman, the name rinderpest would strike horror into people hundreds of years ago. We humans have a list of diseases that can be catastrophic: plague, smallpox, cholera, typhoid, typhus. Cattle had rinderpest, which to them is equally devastating. Nearly 100% of a herd can be wiped out once the infection sets in, with no cure once an animal is infected. Some speculate that rinderpest was the biblical fifth plague of ancient Egypt, wherein the livestock was felled. Symptoms vary with the strain of the virus, but typically there is a sudden onset of high fever, lethargy and loss of appetite, nasal and eye discharge, and rapid, labored breathing. The mouth's discharge turns bloody and necrotic, and the animal develops profuse diarrhea. Most animals die within 6-9 days following the onset of symptoms.
Fortunately, thanks to vaccination, rinderpest is extinct in farm animals today, but the history of the virus is of profound importance. (The name comes from the German word for cattle, rinder, and pest is short for pestilence). Molecular genetic evidence shows that measles, which affects only humans, originated from the virus that causes rinderpest. Some time ago, maybe as short as twelve hundred years, there was a mutation in the virus, allowing it to “jump” from cows to people. It has been firmly associated with humans ever since, leaving a path of destruction and horror in its wake.
The measles virus is technically classified as Morbillivirus. The “morbilli” part of it has an interesting history. Diseases often go by different names depending on time and place. Measles and smallpox have some notable similarities and were thought to be related for centuries. Smallpox was once known by the Latin name morbus, meaning disease. Measles was thought to be related but not as serious and was assigned the moniker morbilli, or smaller disease. The viruses causing smallpox and measles are entirely different, but based on symptoms alone the designation made sense, and the ancient term for measles remains as the name of the viral group.
Around the early 14th century the term measles was first used. It probably arose from the Latin misellus, meaning “wretched” or “miserable.” The proper technical term today for the disease is rubeola, from the Latin word for red, rubeus.
Morbillivirus is a member of a viral family known as the Myxoviruses. Myxo is from the Greek word for “mucus” or “mucin.” Mucin is a protein on the surface of red blood cells to which these viruses attach, causing the RBCs to clump (agglutination). The large group is broken down into two main types, Orthomyxovirus, or “true” Myxoviruses (the primary member here is the influenza virus), and Paramyxovirus, or “alongside” the true virus. Paramyxovirus includes many members, including the measles virus. All Myxoviruses are single-stranded RNA and have a wide range of host animals.
The invasion and infection by the measles virus is a paradox. It is acquired through the respiratory tract, but it is not a traditional respiratory virus. It initially invades lymphocytes and other cells that are part of the immune system, not the cells lining the respiratory tract. The virus does an excellent job suppressing our immune system by invading and killing the cells designed to repel it, activated lymphocytes and dendritic cells. And yet the body can still mount a vigorous immune response to eliminate the virus and establish the immune memory needed to prevent it from ever attacking again. There also is a great disparity in the mortality rate the virus leaves in its wake. There are credible reports of very high death rates in some populations and situations, sometimes reaching 20%, while the mortality rate is low in developed countries of the modern world. Such is the nature of this most unusual viral predator.
The measles virus is a relatively simple appearing creature, small and mostly spherical. It has a single strand of RNA, which is tightly wound in protein. Its coat contains two proteins, F and H, which allow the virus to attach to and penetrate a host cell. Just below these, forming the outer core, is a matrix protein, designated M. Altogether, the virus produces eight proteins.
After entering a susceptible person, the virus is encountered by dendritic macrophages, which carry it to nearby lymphatic tissue to begin building immunity to the microbe. But a funny thing happens during the journey to the lymph tissue: the viral attachment molecule H is strongly attracted to a protein on the surface of activated lymphocytes, CD-150 (also referred to as SLAM, short for signaling lymphocyte activation molecule). Lymphocytes begin their life’s mission being naïve, non-specific, and awaiting chemical instructions on how to develop. Once a lymphocyte, either a T-cell or a B-cell, engages the appropriate foreign material, it becomes active and produces on its surface this CD-150 protein. By bearing it, the cell is constantly exposed to supporter cells that signal it to stay alive. Without it, the cell would die off as most naïve lymphocytes do. Keeping these activated lymphocytes alive is vital to immunological memory. If they are lost, they take with them the ability to mount a vigorous immune response when a pathogen is encountered for a second time.
By invading through the portal of the CD-150 protein of activated lymphocytes, the measles virus invades and kills the cells we need to protect us against previously encountered invaders. This, of course, leaves us vulnerable in the weeks and months following measles infection, and often the disease’s fatal outcomes are due not directly to the measles virus, which is usually controlled, but by the secondary invasion of a pathogen we are no longer able to easily fend off.
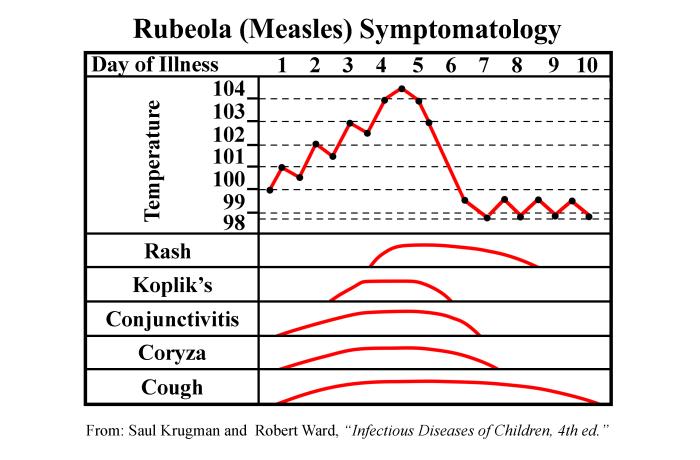
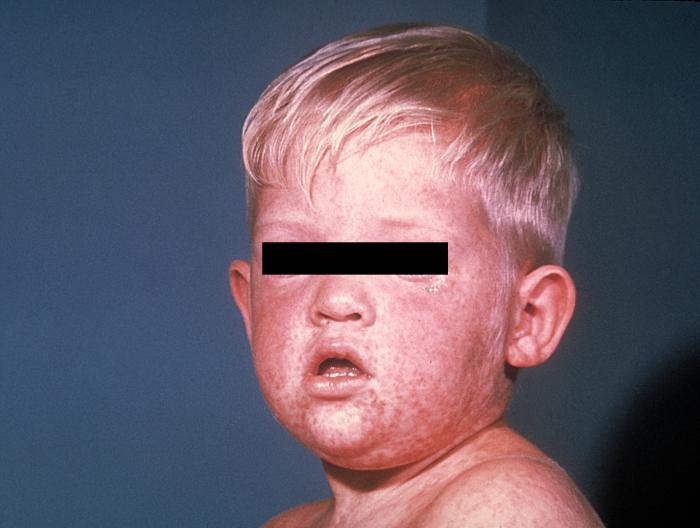
Lymphocytes are mobile, of course, and they can travel to all parts of the body, taking the measles virus with them. As the virus hatches out of its host cells, millions of virions are released throughout the body, leading to a massive cytokine response by macrophages and dendritic cells. This cytokine release gives rise to the symptoms characteristic of measles, starting with the “three C’s”: cough, coryza (runny nose), and conjunctivitis (red, watery eyes). It takes about twelve days, give or take a couple, for the symptoms to manifest. Late in the incubation is the prodromal period, in which the symptoms begin appearing. At first, it looks like a common head cold, but over the next few days, the symptoms are anything but common—most patients, usually children, experience profound muscle aches, prostration, and fever. The fever can be severe, often reaching 106°F. Fever this high sometimes leads to seizures. These symptoms persist for up to a week. The patient is indeed very ill; some require hospitalization. Not long after the onset of fever, the rash appears, first appearing on the back of the neck, then spreading to the trunk, and then to the limbs.
The word pathognomonic comes from two Greek words, patho, or “disease,” and gnomonikos, or one who “knows or judges.” In medicine, the word (pronounced: pathog-no-MON-ic) is usually used to describe a sign or symptom that secures the diagnosis. In the case of measles, the pathognomonic symptom is Koplik’s spots, small whitish spots usually on a reddened background found on the inside of the cheek, adjacent to the second and third molars. They are named after Henry Koplik, an American pathologist who first described them in 1896. (Others claimed to have described them before Koplik, but they were named after him, and so it stands). The spots are rather subtle, and a healthcare worker would likely have to suspect measles and look for them with a flashlight. They appear about two or three days before the rash and are usually gone by the time it appears.
The reason for Koplik’s spots, the characteristic rash, and the respiratory nature of measles is that the virus has not one but two attachment receptors on human cells. The H protein attaches to lymphocytes, an advantageous accomplishment for the virus. But just involving itself with lymphocytes is a one-way street to extinction. The virus must get out of the host and on to the next victim (host). It does this through the second receptor, located on the surface of epithelial cells, designated nectin-4, also called poliovirus receptor-like 4, because polio attaches to the same receptor. Chemically, nectin-4 closely resembles CD150, the receptor on lymphocytes. Nectin-4 is present in all our epithelial cells, so the virus has a lot of places to go, including the skin. This attachment to the sub-layers of the skin and the immune response to it gives rise to the rash. The rash is known as maculo-papular, which is flat, red, and spreading. Pretty much the entire body, including the palms of the hands and the soles of the feet, are covered. But nectin-4 is most prominent in the epithelial cells of the respiratory tract, and that’s where the virus makes its stand. With a severe cough, the virus is spread to the next vulnerable victim.
The measles virus is about the best there is at going from one individual to another. In common parlance, it’s known as a “super-spreader.” One person, with one cough, can spread the virus to numerous people in the same room. The infective dose for measles is very small (very few virus particles are needed to set up an infection), and millions of virions are coughed out from the infected individual. Unfortunately, the virus can cause extensive damage to the epithelial cells in the respiratory tract that it infects, leaving this area wide open for secondary infection by bacteria or other viruses. And, of course, the immune memory system has been laid low. Many of the deaths resulting from measles infection result from this immune suppression along with a secondary infection.
The measles virus is highly efficient and dynamic. It has two points of attack, activated lymphocytes and epithelial cells throughout the body. The characteristic rash that develops late in the disease epitomizes the involvement of the virus: it invades the body from the head and neck down to the soles of the feet. It’s not just the skin that is involved. Just about every bodily tissue, including the brain, are vulnerable to the virus attack. Just how much damage the virus will do to any individual is unpredictable. With the suppression of the immune system and the invasion of numerous tissues, the fate of the disease is like Russian roulette. It could be a straightforward, albeit serious, viral infection that resolves within two weeks or one with a protracted or fatal outcome. There are many reported sequelae following measles, including hearing problems and deafness, encephalitis, pneumonia, and digestive problems.
Historically, there has been an extensive range of reported deaths from measles infection. In some settings, the mortality rate rivaled that of smallpox, over 20% of the population. Today in developed countries, the death rate is one in several thousand infected. One of the chief factors affecting the mortality rate is the presence of another disease or condition that leaves the patient more vulnerable to the measles virus. Known as co-morbidity, the other condition can range from malnutrition to an infectious disease. Getting hit with a rampaging virus like measles when already compromised can be a death blow.
Many bacteria can be grown in a batch of nutrients. Like organisms that grow in sour milk, some bacteria can grow in laboratory nutrients, known as culture media. The media can be carefully selected to provide just the right combination of ingredients to allow the bacterial species to prosper and grow, often displaying characteristics of its species. Viruses, however, need living tissue to reproduce and multiply. Just like the culture media required to grow bacteria, viruses have their favorite types of cells in which to grow, and choosing just the right cells for a particular virus can be challenging. Of course, the cells in the laboratory on which the virus grows must be kept alive, with special attention to preserving the cell line.
One of the pioneers in getting host cells to grow in the laboratory and finding just the right types of tissues suited for a virus was John Enders, one of the great scientific minds of the 20th century. His big breakthrough came in 1949 when he and fellow virologists Thomas Weller and Frederick Robbins got the polio virus to grow earnestly in tissue culture. This feat enabled others, notably Jonas Salk, to produce large volumes of poliovirus, disable it with formaldehyde, and create a vaccine. While Salk received most of the credit for the polio vaccine, Enders, Weller, and Robbins received the Nobel Prize in physiology in 1954 for their landmark discovery.
After the success with poliovirus, Enders turned his attention to the measles virus. Virologists had attempted to cultivate the virus for years but without success. Enders, along with colleague Thomas Peebles, worked out a system of measles virus cultivation. Enders was responsible for the lab work, while Peebles secured the specimens. Peebles traveled to several private boarding schools in Massachusetts to obtain clinical material, taking throat swabs. He then brought the specimens back to the lab, and the swabbed material was inoculated to the tissue culture tubes. One of the first ones from which they cultivated the measles virus in the lab was obtained from an 11-year-old boy, David Edmonton. It was young David’s virus that went on to become the measles vaccine strain, henceforth called the Edmonton strain.
After achieving the growth of the measles virus in the laboratory on human tissue cells, (tissues from discarded placentas from a nearby obstetrics unit were used), the Enders team set about the task of attenuating the virus to make it enter human cells while stimulating an immune response, but without making the person sick. It was a step-by-step process for many generations of viral passages. The measles virus only naturally infects humans and some non-human primates, but Enders was able to get it to grow on chicken embryos. This was the critical step they needed, as putting the virus into a non-hospitable environment would encourage the growth of a mutated virus instead of the wild-type one. If a virus mutates and can grow on non-primate tissue like chicken embryos, it will be selected and survive. The disease-causing strain won’t be able to survive and will die out.
That’s precisely what happened. After years of tedious laboratory work, Enders’ team produced an attenuated measles virus that could grow on chicken embryo tissue. It could still invade human cells but in a much-reduced way. Injecting this virus into people stimulated the immune system to counter it, rendering the vaccine recipient immune, but the virus produced either no discernable disease or a very mild one. As was customary of the time (the late 1950s), the experimenters inoculated themselves and measured their symptoms and antibody response. While they all had had measles as children and were already immune, they showed that the antibody response to the attenuated measles virus was vigorous, and there were no noticeable side effects.
Of course, the live measles vaccine had to be verified for its effectiveness in the population. The researchers decided to administer the vaccine to a group of youngsters living in an institution for disabled children. Such institutions were pretty common at the time. Chosen was the Walter E. Fernald State School near Waltham, Massachusetts. The children housed and cared for there had mental or physically challenging disabilities. As happens so many times, well-meaning people can see an event in very different ways. To some, giving an experimental vaccine, a live one at that, to medically compromised children was an act of barbarism, a sure sign that the researchers considered the lives of such children minimal and expendable. The other opinion espoused by the researchers was that such children were at exceptional risk of severe complications or death should a measles outbreak occur. Since they were very confident in the vaccine's efficacy and safety, they were the very group that needed to be protected.
In the late 1950s, research standards weren’t what they are today. Often experiments were carried out without the subjects’ knowledge, or their tissues or body fluids used unknown to them. The measles research team under Dr. Enders was committed to scrupulous scientific ethics. Dr. Samuel Katz, the MD administering and monitoring the vaccine, ensured everyone involved had their parents fully informed and consent forms signed. In 1960, the experimental vaccine was administered to children in the Fernald boarding school, and follow-up studies were conducted each day. Blood samples were drawn to test for antibodies, and throat swabs were taken to search for the virus. Antibody levels were high, and the virus was undetected, a very good outcome. Based on these results, further testing was done around the United States with the same results. The measles vaccine had become a reality.
(The appropriate testing of the disabled children at the Fernald school was born out a couple of years later when a natural measles epidemic swept through the school. While recently admitted and unimmunized children became very ill with several deaths, the children given the experimental vaccine all showed no illness and no deaths).
The live, attenuated measles vaccine began to be used in the early 1960s. It wasn’t perfect. Some children, maybe one or two in ten, developed some fever and discomfort a little over a week after vaccination. It was much better than wild-type measles during an epidemic, but vaccines with very few symptoms are the ideal. At Merck Pharmaceuticals, Maurice Hilleman and his crew further developed the attenuated Edmonton strain with many passages until it was more suitable than the original vaccine strain. The Merck strain has been combined with live attenuated mumps and rubella viruses to give us the present-day live tri-vaccine of measles-mumps-rubella, commonly referred to as MMR.
The alteration in the virus was marvelous. After passage through several different tissue cell lines, the chief attachment protein of the measles virus went from an affinity for the CD-150 receptor of activated lymphocytes to one known as CD-46. The latter is present in all human cells except red blood cells. The serum complement system is a potent destroyer of cells, including human. To prevent collateral carnage from activated complement, our cells are equipped with the CD-46 molecule, which effectively destroys the C3 component of complement, leaving our cells safe. At the same time, C3 does its work on invading bacteria. By attaching to the CD-46 receptor, the measles virus can still get into the host cell, but, for some reason, it is much less active, and the immune response to it is vigorous. Interferon is liberally released. The host cell undergoes apoptosis and dies, taking the virus with it. The immunized patient experiences little or no physical awareness of the virus. It’s an ideal vaccine.
Another mystery of measles is how immunity to it lasts a lifetime. Our immune system has an excellent memory, but 60 or 70 years is a long time for T and B lymphocytes to hang around. The presence of CD-150 on activated cells, and its effect on memory, is helpful. Still, other infectious diseases require booster shots from time to time to keep immunity thriving. Tetanus shots, for instance, should be given every ten years or so. But immunity to measles, whether acquired naturally or through vaccination, seems to last as long as the patient. One unproven theory is that the virus is sequestered in some cells, perhaps lymphocytes, and emerges from time to time only to be confronted vigorously by antibodies, enhancing the immune response, something of a natural booster shot. Whatever the reason, the measles vaccination has been incredibly good for humanity, at least for those fortunate enough to get it.
The word “patrician” refers to a person of very good background, education, and refinement. It fits Dr. John Enders perfectly. Born into a wealthy family in Connecticut, he tried several ventures until he became enraptured by the laboratory work of his college roommate. He became a virologist and worked his way to the forefront of the development of one of the great scientific accomplishments of the modern age, the cultivation of viruses on laboratory-grown tissue cultures. Before this work in the 1940s, it was necessary to experiment with viruses strictly in animals, which was clearly a burdensome task. Ender’s work led the way to the near eradication of two horrible diseases, polio and measles, and it enabled others to use his laboratory’s techniques to work toward eradicating and diagnosing other pathogens. Truly an amazing accomplishment.
By his colleague’s accounts, Dr. John Enders was an avuncular figure, always helpful, supportive, and encouraging. He frequently engaged in light conversation and held his employees in high esteem. The field of science can, in some ways, mimic others, with some of its members being a rapacious sort, quick to grab credit, glory, and the spotlight. This wasn’t Dr. Enders, a kindly man who made some of the greatest discoveries of all time but whose name is virtually unknown to today’s populace. His work has saved the lives of tens of millions of people and the terrible suffering of hundreds of millions more. He received a Nobel Prize along with Thomas Weller and Frederick Robbins in 1954, but the world owes him an even deeper debt of gratitude.
Measles is rare in the developed world. Most people today don’t appreciate the magnitude of its ferocity. In the pre-vaccine era it attacked mainly children. It was estimated that by age 15, over 90% of the population would have contracted measles. Because of this, it is usually classified as a childhood disease alongside chicken pox, whooping cough, mumps, roseola, and German measles (rubella). Measles (rubeola) tends to get minimized historically. But it was, and is, a wretched disease, with a fever of up to 106° for up to a week, with seizures, hallucinations, and physical prostration. This is followed by a rash over the entire body that results in incredible itching. Add to that the suppression of the immune system for several months after the infection, leaving the patient vulnerable to potentially serious secondary infections. Measles is not, by any measure, kid’s stuff.
The term herd immunity has been known to epidemiologists for many years but has become more commonly recognized in the popular lexicon in the past few years. Simply stated, infectious diseases transmitted from person to person, usually by the respiratory route, will not prosper and will quickly die out if a large percentage of the population is immune. When most of the people on the Faroe Islands had contracted measles in 1846, Peter Panum made the astute observation that the epidemic was over, and no new cases were observed. Such is the case in large cities, as the virus attacks only humans, and when all potential victims are either immune or dead, the virus has no place to go. Historically, measles came around every 2 to 4 years, when young children were old enough to be mobile and no longer protected by their mother’s antibodies that they acquired in the womb.
Through much research, it has been determined that the percentage of people needed to be immunized against measles to achieve herd immunity is around 92%. That’s a very high number, but one that reflects the highly infectious nature of the virus. If a mainly enclosed population does not have 92% of its members immunized, and measles is introduced, trouble, much trouble, ensues.
The rash of measles typically begins around the fourth day
of illness and often encompasses the entire body. (PHIL)