Tuberculosis
Consumption
Macrophage seeking
Malevolent red snappers.
The dreaded white plague.
Patrick Brunty was born in Northern Ireland on St. Patrick’s Day, 1777, to a family of poor, illiterate farm workers. At an early age, Patrick showed academic prowess, and when he came of age, he was awarded a scholarship to Cambridge. Perhaps he felt his Irish heritage would be disparaged, so he changed his last name to Brontë, being particular about the insertion of the umlaut over the ë to lend it a touch of class. Little did he realize at the time that the name Brontë would become one of the most famous names in 19th-century English literature.
Patrick married Maria Branwell in 1812. They went on to have six children, five girls and a boy. After becoming an Anglican minister, Patrick accepted a post in the poor industrial community of Haworth in 1820. Theirs was apparently a happy family, with the father Patrick the primary educator of his children. Unfortunately, tragedy stalked the house. Wife and mother Maria, a bright, cheerful, well-educated woman, died of cancer at the age of 38. Her sister Elizabeth came to live with the family. Then the two eldest daughters, Maria and Elizabeth, died at ages 11 and 10, respectively. The three remaining daughters, Charlotte, Emily, and Anne, became very talented writers of high notoriety, their works greatly influencing English literature. But they too would experience early deaths, Anne at age 29, Emily at 30, and Charlotte at 38. Their brother Patrick also died early, at age 31. The father, Patrick, outlived his wife and all his children.
What struck most of the Brontë family was a disease that has been with human beings for as long as there have been human beings. Ancient writings from several different cultures refer to it, and examinations of skeletons of early ages attest to its presence. But with the advent of the industrial revolution in the early 19th century and its attendant overcrowding, poor ventilation, poor nutrition, and lack of sanitation, the disease tuberculosis exploded in numbers, killing millions indiscriminately.
TB has had several names throughout the ages, some associated with two of its major symptoms, weight loss and hemorrhage. The Greeks referred to it as phthisis (pronounced “thigh-sis”), from their word for “wasting away,” phthinein. A more modern term was “consumption” because the disease " consumed " the patient. Another common name was “the white plague,” owing to the facial pallor due to anemia. The term tuberculosis, from tubercle, was first used in the early 1800s when it was discovered that the disease is not confined to the lungs but can infect other tissues. The nodules formed on infected tissues resembled “tubers” (fleshy outgrowths). The
“-cle” means miniature.
The organisms that cause TB are members of a genus with an enormous number of species, Mycobacterium. It’s an odd sort of bacterium with a peculiar name. The prefix Myco refers to fungus or toadstool, clearly having nothing to do with bacteria. The reason for the term is that when grown on artificial media in the laboratory, the colonies the organism forms resemble those formed by some fungi. In nature, there are close to two hundred species of Mycobacteria that inhabit many and varied habitats, often in dirt and water. Most have nothing to do with infectious diseases.
But one group of Mycobacterium many millennia ago became associated with humans and animals and has since become entirely dependent on them for their existence. The main species of this group that infects humans is Mycobacterium tuberculosis. A half dozen other closely related species can infect animals and cause the same symptoms in humans, but they are rare. We often use the term Mycobacterium tuberculosis complex to include these other species.
Mycobacterium is a unique, disparate group, different from other bacteria in several ways. The chief difference lies in their cell wall, which contains several distinct features. Nearly all bacteria have the substance peptidoglycan in their cell walls. It is a complicated structure combining carbohydrates (glycan) and peptides, short strands of amino acids (peptido). Peptidoglycan (PG) is a strong mesh-like structure that gives the bug its shape and keeps it from coming apart because of the internal pressure of the cell. The PG layer of mycobacteria is like that of typical bacteria but with a few tweaks. Those minor variations allow it to connect to the “secret weapon” of mycobacteria, a thick, wax-like substance called mycolic acid. Gram-negative organisms like E. coli have lipids in their cell wall, but mycobacteria take the lipid content to a much higher level. This thick layer on top of the bacterium’s cell wall serves several functions. For one, it enables the organism to stay alive inside the cell designed to kill it. It also prevents the entry of substances that may harm it, such as antibiotics.
TB is a respiratory infection, so it would be easy to liken it to the viruses that infect by the same route. But TB is much different than viruses like influenza and coronavirus. It’s a bacterium and therefore much larger and heavier than the viruses. Also, TB doesn’t infect the upper airway like the throat, larynx, or trachea. Its target is the lungs themselves. So, TB is much harder to “catch” than the viruses are. Typically, one must inhabit the same confined space as the infected person who is actively coughing and expectorating aerosols containing the organism. Spread of the infection occurs almost exclusively indoors, mainly in smaller confined rooms. After the expulsion of the organism from an infected individual in the form of an aerosol, the moisture surrounding the organism evaporates, leaving the organism suspended and wafting in the air. The more organisms in a room and the longer people congregate there, the greater the chance of its spread to an uninfected person.
Even when the organism within the aerosol is inhaled, a vital protective mechanism is in place to repel it. The mucus in the respiratory tract traps them, and the constantly beating cilia on the epithelial lining expel them. When functioning properly, it is a very efficient system. But, of course, this elegant scheme can break down when overburdened by environmental factors, like lots of dust, grit, smoke, and other assaults. This can open the door for some of the inhaled TB germs to make their way straight to the lungs.
Once the organism gets to the alveoli and surrounding tissues of the lungs, it encounters the pulmonary macrophages, cells tasked with keeping the lungs' inner workings free of foreign material, both microbes and inanimate substances. We each have billions of these cells constantly employed in healthy lung tissue, and they are very good at their job. Unlike our other vital organs, the lungs are exposed to the environment, and keeping them sterile is a full-time job.
Most bacteria that enter the lung tissue are harmless commensal organisms that are dealt with in quick order. The macrophage engulfs them within a phagosome, a lysosome combines with the phagosome within the macrophage, and toxic granules kill the organism. Some pathogenic bacteria have devised mechanisms to avoid the engulfment by the macrophages. Most notable is the organism Streptococcus pneumoniae, which, as its name suggests, is a common cause of pneumonia. It has a thick capsule of carbohydrate designed to make the job of engulfment by the macrophage more difficult, giving it time to set up an infectious process. Other organisms that cause pneumonia have similar weapons.
Mycobacterium tuberculosis (MTB) is different. Instead of avoiding the macrophages designed to kill them, MTB seeks them out, and its heavy lipid, wax-like outer membrane makes it very easy for the macrophages to engulf them. But once inside the macrophage, within the phagosome, the bacterium gets the upper hand. Normally, the lysosome fuses with the phagosome containing the bug, and the organism’s death results. But for MTB the result is altogether different. The lipid layer of the organism prevents the fusion of the lysosome with the phagosome. In fact, the phagosome itself unravels, releasing the organism into the cytoplasm of the macrophage, where it can multiply unfettered. Instead of being a killing machine, the macrophage is now an incubator for cultivating more Mycobacteria.
In addition to killing an invading organism, the macrophage sends chemical signals alerting other immune system components that trouble is afoot. Chief among these are interferon-gamma (IFN-gamma) and tumor necrosis factor-alpha (TNF-alpha), which attract other macrophages into the area. Inside the infected initial macrophage, the organism is multiplying, eventually killing the host cell. The freed organisms can now infect the attracted macrophages with the same fate as the initial one, multiplying with impunity. This sequence of events continues until many new organisms are produced.
Meanwhile, other members of the immune system are alerted by the chemical signals of the infected cell, chiefly T-helper cells and other macrophages. They close ranks around the growing number of Mycobacteria inside the infected macrophages, forming a cellular capsule around it. Mycobacterium is not motile, so they stay in a confined location. This surrounding group of cells, along with the fibrin and other matrix material accompanying it, is called a granuloma, since the macrophages involved contain granules. The suffix “oma” refers to its resemblance to a tumor. Other entities can lead to the formation of granulomas: other bacteria, fungi, parasites, and even some human tissue that gets in the wrong place. But granulomas are characteristic of TB and are a very helpful diagnostic tool.
In tuberculosis the center of the granuloma becomes necrotic and assumes a dull white appearance, something like soft cheese. The Latin word for cheese is caseus, and granulomas with this type of necrotic center are referred to as caseating granulomas.
Mycobacterium tuberculosis is a very slow-growing organism. Some bacteria can reproduce every twenty minutes in the proper culture media. MTB takes around twenty hours. Being non-motile, it depends on the immediate environment for its nutrients, and since it is stuck in the middle of the granuloma there isn’t much available. So it enters a state of quiescence, still alive but not doing much. This is the best outcome for the afflicted patient, having the bacterium in the latent state. As long as there are plenty of active CD4 lymphocytes this latent state persists, usually for many years.
Problems arise when the person’s immune system breaks down. There are many causes for such a reduction in the immune response, some more impactful than others. Malnutrition, other diseases both physical and mental, medical therapies that affect immune status, continued exposure to harmful environmental conditions, and genetic alterations in some of our immune constituents play a large part. Getting infected by the organism isn’t a sentence of overt tuberculosis. Other conditions play a significant role.
The major problem in tuberculosis is the formation of a cavity in the lung. When confined to an individual granuloma, that organism isn’t very damaging. It assumes a latent stage and transmission to other individuals is not a concern. If the granuloma breaks down, however, the organisms leak out into nearby bronchial airspace and begin to proliferate wildly with the abundance of oxygen present in the lung. A cavity forms within the area. While the number of bacteria in a granuloma is in the low hundreds, the number in a cavity is in the tens of millions. Tissue destruction occurs, and at this point the prognosis is poor.
Sometimes the organism can enter the bloodstream and be carried throughout the body, potentially infecting several organs outside the lungs. The lungs, too, can be infected over again in multiple locations. When first observed in the early 1700s, the small growths throughout the organs were described as resembling millet seeds, and the term applied was miliary TB. Multiple organs, including the bones, can be infected, and it is a grave medical situation.
Because of its means of infection, inside macrophages and sequestered in granulomas, the humoral defenses of antibody and complement have little effect on the course of illness. Antibody is produced, but it is inconsistent. A little is made against the organism's cell wall, but more against the interior components and proteins, which, by its nature, gives an inconsistent pattern. The overwhelming immune response to infection by Mycobacterium tuberculosis is the cellular response led by macrophages and T-cells. Fortunately, we have a test to utilize this activity to aid in identifying those infected.
The tubercle bacillus was first grown in culture and identified in the laboratory of German researcher Robert Koch in the 1880s. It was a monumental achievement, and Dr. Koch received the Nobel Prize for his work in 1905. Shortly after the discovery of the organism, a skin test was devised using organisms that were killed, purified, and injected into the skin. People who had the organism in their body gave a reaction of swelling and induration (hardening) at the site of the injection. The original preparation was very crude, and it was improved upon by several workers, most notably French investigator Charles Mantoux in 1907. Even his better version needed revision and standardization, and it wasn’t until the 1930s that a reliable preparation was available. It goes by several names, including the Mantoux test, PPD (for purified protein derivative), and tuberculin. The material is injected into the dermal layer of the skin, usually between the crook of the elbow and the wrist. If the person being tested harbors the organism, the injection site becomes swollen and hard after two or three days. (Redness doesn’t count).
When injected into the skin, the material from the organism is internalized by macrophages in the area. Inside the macrophages it is processed, and small parts of the material are placed upon an MHC molecule, prominently displayed, and transported to the macrophage’s surface. T-cells that have been previously activated by the presence of the organism in the body recognize the microbe’s antigens being displayed by the macrophage, proliferate at the site, and the tissue damage ensuing results in the creation of the hardened area at the site of the injection. It takes about 2-3 days for this to take place. Done properly, the TB skin test is usually very reliable.
Another way of detecting T-cells that have been activated by Mycobacterium tuberculosis is to draw a blood sample and expose the lymphocytes in the sample to antigens of the organism. The T-cells’ first response is to elicit interferon-gamma, which can be detected by chemical analysis. The blood must be tested shortly after being drawn to ensure the lymphocytes remain alive. The main advantage over the skin test is that no follow-up visit is necessary. For the skin test the tested individual must return within 2-3 days to have the test read. Both tests are subject to false positives and negatives, but, in the big scheme of things, they are very useful tools in determining the presence or absence of Mycobacterium tuberculosis within the tested person.
In developed countries, most people don’t need to be routinely tested for the presence of TB. Healthcare workers who may have been exposed, laboratory workers, arrivals from countries where the disease is endemic, and anyone known to have been around someone diagnosed with tuberculosis are routinely tested.
The unique properties of the organism make laboratory work challenging. The bug is slow growing, has a very peculiar cell wall making it hard to stain, and it infects deep in lung tissue, not the upper respiratory tract. Specimen quality is very important. Applying routine laboratory techniques is insufficient to detect the organism; specialized procedures are required.
A representative specimen is critical to all laboratory testing. For pulmonary tuberculosis, the easiest specimen to obtain and the least expensive is the expectorated sputum sample. That’s sputum, from deep in the lung, not saliva. Merely spitting into a specimen cup is inadequate if all that comes out is colorless, foamy saliva. What’s needed is the gunky stuff from down below. Often the best way to obtain this better material is when the patient first wakes in the morning; sputum tends to accumulate during sleep. Sometimes a fine mist of an aerosol can be breathed in to loosen the material and help cough it up. If this doesn’t work, a tube can sometimes be inserted down the trachea and material aspirated.
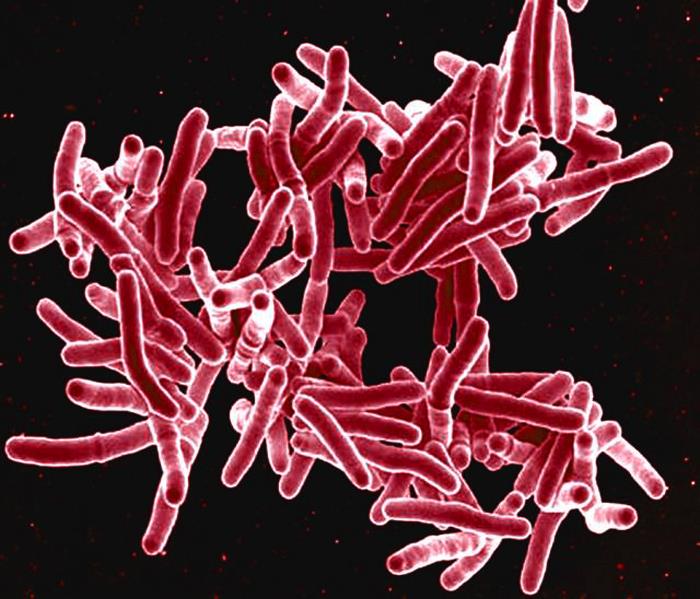
The routine stain applied to clinical material to observe bacteria is the Gram stain. Mycobacteria don’t stain very well with this technique, however, often giving a speckled appearance or no staining at all. Particular to mycobacteria, though, is the fact that their cell walls make them resistant to the penetration of organic solvents as well as acid. This fact has given rise to the term “acid-fast,” and they are collectively referred to as acid-fast bacilli. A staining technique known as the Ziehl-Neelsen stain is routinely done in clinical laboratories to detect the presence of mycobacteria. The bacteria do not divide evenly side by side or end to end like other organisms; they just sort of randomly snap off the mother bug. Since they stain red from carbo-fuchsin application, lab folks call them “red snappers.”
Most mycobacteria grow very slowly; the TB bacillus takes around three weeks to show a colony on culture media. Other bacteria in the sample, if it was collected through the mouth, grow much faster and will destroy the culture media before the mycobacteria have a chance to grow. To compensate for this, a sputum sample can be treated with two ingredients, one to digest and homogenize the sample by neutralizing the mucus, and another to kill the competing bacteria while sparing the mycobacteria. For the former, n-acetyl-cysteine is usually used (NAC), and for the latter, sodium hydroxide (NaOH) is commonly used since it doesn’t kill mycobacteria at the concentration employed.
The smear and culture for mycobacteria, usually just called AFB for acid-fast bacilli, is not perfect but still can be very helpful in both establishing a diagnosis of active TB, and ensuring that a patient is not infectious during the early stages of therapy. Smear and culture are of no use in diagnosing patients with latent TB because they are not shedding organisms. That’s where skin testing and interferon-gamma tests come in.
About 90% of people infected by Mycobacterium tuberculosis do not show signs of TB. Their cellular response to the infection is sufficient to keep the organism at bay. Some people, though, are overwhelmed by the infection. Also, changes in immune status, such as those which occur with malnutrition, immunosuppressive medications, exposure to noxious chemical aerosols, or infection with human immunodeficiency virus (HIV), can lead to the emergence of tuberculosis.
The most common symptoms of active TB are not universal but, when present, can point strongly to the diagnosis. One is unexplained weight loss over a month or two time period. Another is continuing night sweats, and a third is blood in the sputum. These are not unique to TB, but they certainly lead the physician to look in TB’s direction. X-rays are also very distinctive in most cases.
Throughout human history the diagnosis of TB was close to a death sentence. Certainly, there were attempts at treatment, but folk remedies and other such pursuits were futile. A new approach to treatment that often proved successful was introduced in Europe in the early to mid-1800s, the sanatorium. For a long time, it had been the practice of wealthier people to go to health spas, seeking rest, relaxation, and more than a little pampering. Sometimes mineral springs were involved. This type of environment inspired the creation of sanatoria, and they began to multiply toward the end of the 1800s and early 1900s when the cause of TB became known. (The words saniTARium and sanaTORium are today often used interchangeably. But the clinics for treating TB were referred to as sanatoriums. The Latin root of the word is sanitorius, meaning “health-giving.” The root for sanitarium is sanitas, meaning health. Changing a few vowels was done to distinguish the two).
In the era before antibiotics, sanatoriums offered the best treatment available when used correctly. Perhaps most importantly, they were located in remote areas, thus isolating infected people and preventing disease spread. While it is impossible to prove, the treatment of tuberculosis with fresh air, good nutrition, sunshine, and rest has a lot going for it.
Most TB patients contract their infection by being indoors with poor ventilation and particulate matter in the air. Fresh air, with open windows and doors with screens, minimizes the amount of extraneous matter entering the lungs, allowing the pulmonary macrophages to concentrate on fighting the infection.
Proper nutrition, including protein, vitamins, and fluids, provides the foundation for the immune system to react energetically to the infectious process and, just as important, to the rebuilding process. TB damages lung tissue: it needs to be repaired efficiently.
Vitamin D activates the genes responsible for releasing cathelicidin, an anti-microbial compound found in macrophages. Cathelicidin attacks the membrane of bacteria such as Mycobacterium tuberculosis and attracts T-cells and macrophages to the infected site. For a long time, it was known that cod liver oil was helpful in treating TB in some patients. This is because it is rich in vitamin D. For patients who were deficient in vitamin D, supplements like cod liver oil boosted the immune response.
Vitamin D is activated by sunlight. Having enough vitamin D in the body (over 30 nanograms/ml) and having it activated by sunlight is a good thing. Sunlight quickly dries out the droplet nuclei of expectorated respiratory material, and the UV portion kills the bacteria.
Rest and lack of exertion remove the strain on the lungs, allowing them to heal faster. Mycobacterium tuberculosisis a strict aerobe, requiring oxygen for growth. Most infections occur in the upper lobes of the lungs. Reclining reduces oxygen tension in the upper lobes, presumably slowing the organism’s growth.
With the introduction of anti-microbial agents to treat TB in the late 1940s and early 1950s, sanatoriums were suddenly out of favor. And it’s no wonder. Patients had to leave their homes and live for many months in a remote place that was, for all intents and purposes, like a prison. They were confined to bed the entire time, with only reading material for entertainment. The mental strain was considerable. But there were undoubtedly many successes in the treatment of TB in the sanatoriums, though it is impossible to assess just how many recovered there versus how they would have done if treated at home.
Albert Schatz made one of the greatest scientific discoveries of all time. He was born in the northeastern United States in 1920 to a family of farmers who had emigrated from Europe. He planned on becoming a farmer as well, and to edify his knowledge of the subject he entered one of the top agricultural schools of the country at the time, Rutgers University in New Jersey. It just so happened that one of the pre-eminent researchers in soil microbiology, Selman Waksman, was doing research at Rutgers. Albert, who graduated at the top of his class, was attracted to the work the Waksman team was doing.
This was the 1940s, and with the war raging, Albert was drafted into the U.S. military. Because of his laboratory experience, he was assigned to a medical laboratory unit in Florida. There he became acquainted with human pathogens and their cultivation. After injuring his back, he was discharged from the military and returned to pursue his doctorate at Rutgers. Upon returning, he found that the laboratory was vigorously searching for new antibiotics. The university’s expertise was soil microbes, and after the discovery of penicillin, it was felt that that was a fruitful avenue of research. In fact, the Waksman team had discovered three antibiotics from soil bacteria that killed Gram-negative bacteria, something penicillin didn’t do. Unfortunately, they were too toxic for human use, but the door was open for further inquiry.
The genus Mycobacterium is soil-dwelling. While M. tuberculosis is confined to humans, its soil relatives live out their existence in environments shared with other soil microbes. Researchers at the Mayo Clinic in Minnesota suggested to Selman Waksman that, given his research department’s expertise in soil bacteriology, it might be worth trying to find an antibiotic from a soil bacterium that interfered with the growth of Mycobacterium tuberculosis. Given the danger of working with the organism in the laboratory, Dr. Waksman opposed the idea. But Albert Schatz was intrigued and volunteered to take on the project for his graduate studies.
Albert was given some laboratory supplies and relegated to the basement, where he spent the better part of a year. Often sleeping on a wooden laboratory bench and equipped with a few amenities, he spent most of his time down there. But it paid off. Working with two women assistants, Doris Jones, and Elizabeth Bugie, the team was able to report in 1943 that a substance isolated from the soil bacterium Streptomyces griseus killed both Gram-negative and Gram-positive bacteria and, in fact, seemed to be effective against a strain of Mycobacterium tuberculosis. Subsequent testing in mice bore these results out, and the new antibiotic was sent to the Mayo Clinic for testing in humans. It proved a phenomenal success. A new era of antibiotic treatment for tuberculosis had begun. The antibiotic was called streptomycin.
The chemical company Merck, with facilities in both the U.S. and England, put its full resources into the development of streptomycin, and by the dawn of the 1950s it was the treatment of choice for TB. But there were problems. For one, streptomycin must be administered parenterally, that is intravenous or intramuscular; it cannot be taken orally. And unlike the treatment for other bacterial infections like strep throat or urinary tract infection, treatment for TB takes several months, so patients had to be hospitalized or in constant contact with doctors and nurses. Also, streptomycin can display adverse side effects like dizziness and unsteady feeling, hives on various body parts, and nausea and diarrhea. These are more apparent when the drug is administered over many months. Worst of all, the organism MTB can develop resistance during therapy. But all in all, the introduction of streptomycin lent hope to many for whom death was likely.
A notorious side story to the development of streptomycin was the behind-the-scenes intrigue of the people involved. Selman Waksman was the laboratory director in which the discovery was made. He was a widely recognized authority on the subject of soil bacteria. Albert Schatz, at the time of the discovery, was a graduate student working under the direction of Dr. Waksman. It was Schatz who camped out in the lab basement, inches away from a deadly bacterium for months on end. It was his relentless effort that brought the discovery to fruition, and his name was first on the first paper published on the matter. But all credit went to Dr. Waksman, who would receive the Nobel Prize for the discovery. Albert Schatz’s name was never mentioned or acknowledged. Nor were the names of the two women who assisted him with the research. Not only that, but Dr. Waksman had also persuaded Albert to forego all royalties for the discovery, those in turn going to a foundation at Rutgers University and ultimately benefitting Dr. Waksman. Litigation ensued, and the entire affair became a messy blight on one of the most important discoveries of human history.
Streptomycin attacks the protein synthesis of bacteria by attaching itself to a portion of the bacterial ribosome. The main bacterial ribosomes are designated as 30S and 50S. It is the 30S ribosome to which streptomycin binds, disrupting the construction of growing proteins. Because of its chemical structure, streptomycin is classified as an amino-glycoside. It was the first of this class discovered. The group is used today mainly in treating infections caused by Gram-negative bacteria. The aminoglycosides do not by themselves enter the cytoplasm of bacteria. They must be actively transported in, and this transportation step leads to most of the resistance seen in the group.
With the end of World War II and the progress made in developing the first antibiotics, sulfa, penicillin, and streptomycin, much effort was put into finding new drugs. The realization that the first anti-microbial discovered, sulfadiazine, had been formulated many years before its application to infectious diseases prompted the search for similar previously created but unappreciated compounds. Two were found to combat TB: Para-aminosalicylic acid and Isoniazid. To make it easier to say, the names were reduced to letters, PAS and INH. (INH refers to the drug's chemical name, IsoNicotinic acid Hydrazide). In the early 1950s, these two, along with streptomycin, were the standard treatment for TB. Unfortunately, resistance to each developed rather readily in the slow-growing bacteria, but it was found that cure rates were much higher when they were used in combination, a practice that continues today.
Other antibiotics were discovered through the years, including rifampin and ethambutol. A standard course of treatment for active TB today uses four drugs in combination, rifampin, isoniazid, pyrazinamide, and ethambutol, commonly referred to as RIPE. Other situations, such as positive skin test without symptoms or those at high risk, have different regimens.
However, a major problem has been encountered worldwide with the development of drug-resistant strains. Some TB bacteria are multi-drug resistant (MDR), defined as resistance to both rifampin and isoniazid. Extremely drug-resistant strains (XDR) are resistant to those two plus two others. Some choices of drugs remain, but they are less effective. An important reason for the emergence of these drug-resistant strains is the partial treatment of the disease. Some patients take their medication for abbreviated periods, either through neglect, fear of side effects, or non-availability of the drugs. This allows the bug to develop resistance, as those strains are given time to multiply when the drug is discontinued. These resistant strains can then be spread to others, with the initial infection due to a resistant strain.
There are several words that best describe the vaccine for tuberculosis: confusing, bewildering, perplexing, baffling, disconcerting, complicated. Sometimes exasperating applies. Even the name of the vaccine is unusual, just the three letters BCG, which don’t suggest the targeted disease like most vaccine names do. The vaccine has been in use for a hundred years, but many questions remain about the extent of its effectiveness.
In the early 1900s, it was known that tuberculosis was caused by the bacterial organism Mycobacterium tuberculosis. It was also known that cattle had a similar disease, and the organism could be found on the cow’s udder. Two French researchers working at the Pasteur Institute in Lille, France wondered if the phenomenon observed in smallpox could apply here- could a strain of tubercle bacilli infecting cows be used to vaccinate humans with minimal side effects? In 1908 Albert Calmette and Camille Guerin began passing a bovine isolate of TB on successive culture plates. In 1913 they felt it was ready for human trials, but World War I intervened. The vaccine had to wait until 1921. It was called “Bacille Calmette-Guerin,” BCG.
During the 1920s BCG was mainly given to infants by the oral route. It proved to be safe. The questions were about its effectiveness. Those questions persist today.
Putting a huge damper on the use of BCG was the infamous “Lubeck Disaster.” In Lubeck, Germany in 1930, infants were routinely administered the BCG vaccine by the oral route. Tragically, the vaccine material was contaminated with viable pathogenic Mycobacterium tuberculosis in the laboratory in which the vaccine was prepared. Seventy-three infants died, and another 135 were sickened but recovered. The tragedy cast a pall over the use of BCG. An investigation showed the means by which the vaccine strain was contaminated with viable virulent organisms, but questions remained. Indeed, many today who question vaccination in general point to the Lubeck Disaster.
Eventually, the BCG vaccine came to be used again, but there have always been questions about its effectiveness in preventing tuberculosis. Studies have varied significantly in their conclusions. There are several reasons for the confusion:
BCG doesn’t prevent most people from contracting Mycobacterium tuberculosis. The best that can be hoped for is the disease won’t progress as rapidly.
There are over two hundred species of mycobacteria, most of which live in the soil. There is some evidence that exposure to a wide range of mycobacteria will prime the cellular immune system to be more effective in combating Mycobacterium tuberculosis should it infect. Perhaps some people, because of where they live, have already acquired a low level of resistance to TB regardless of BCG administration.
There are four strains of BCG bacteria in use in the world today. Comparing one strain’s effectiveness against another can be tricky. Also, immunity to TB depends on cellular immunity, with lymphocytes and cytokines playing a significant role. The genetics of people within a population can render some groups more susceptible to TB than others in a different geographic location, confounding conclusions about a vaccine that is only partially effective in subduing the disease.
Infestation with parasites can alter a person’s immune responses. In areas of the world with a greater likelihood of TB, there is often a greater number of people infested with parasites. The net effect on the immune system and its response to infection by Mycobacterium tuberculosis is difficult to quantify when the parasite burden is high.
These and other questions abound. Each country of the world makes its own decision about the recommendation of vaccination against tuberculosis with BCG. The United States has never recommended its use. Other countries do. There is no doubt that BCG has saved millions of lives and prevented untold misery. One thing that always must be remembered, BCG is a live vaccine, and anyone receiving it will test positive with a tuberculin skin or blood test.
In the U.S. and other developed countries tuberculosis is no longer endemic. Most of the cases that are diagnosed originate in other countries. Diagnostic tools and therapy, along with vigorous public health intervention, help keep it under control. But worldwide, it remains a scourge. Well over a million deaths are recorded yearly, and millions more have their lives severely impacted by the disease.
Mycobacterium tuberculosis, the agent causing TB, stains red with the acid-fast stain (PHIL)