Lyme Disease
Lyme Disease
Infection by Deception
From invertebrate
To unsuspecting victim.
Spiral predator.
Science prides itself on being orderly. Chemical names, abbreviations, atomic number and molecular weight, all arranged neatly on the periodic table of the elements. Every living thing given a scientific name, first the genus, followed by the species. Proper names assigned to every bone, muscle, and visceral organ of the body. The discipline of science demands exactitude since communication between workers is vital to advancing knowledge.
But the naming of infectious diseases takes a rather different course. Some diseases have names that have been around since ancient times, others more recent. Quite a few have more than one name. More recently, some diseases are nearly always referred to by an acronym rather than the proper name. It’s hardly a neat and orderly process, but somehow it works.
Diseases that have been well recognized for centuries carry their traditional name with them. Rabies, tetanus, malaria, smallpox. They are often assigned a more scientific term, but the common name is usually employed. Variola is usually referred to as smallpox, rubeola as measles.
Often the species of the microorganism responsible for the disease is discovered well after the ailment has been described, and the bug is assigned the name of the disease. Examples are Clostridium botulinum, Bordetella pertussis, Corynebacterium diphtheriae, Neisseria meningitidis, and Neisseria gonorrhoeae. (One great mistake here was an organism thought to cause influenza was given the name Haemophilus influenzae. It turned out it doesn’t actually cause the disease. Oops). Sometimes it’s the other way around, when the organism is known, but the disease description follows. Clostridium difficile was known before the disease it caused came to be known as C diff (more properly pseudomembranous colitis). The genus Campylobacter was just an obscure organism many years before its role in the common intestinal disorder campylobacteriosis was described.
Oftentimes several different organisms can cause the same disease. Pneumonia, meningitis, urinary tract infection (UTI), upper respiratory tract infection (URI), gastroenteritis, and athlete’s foot all can be caused by several organisms. Sometimes, the species name of the organism shown to be the cause is used as an adjective, such as pneumococcal pneumonia or meningococcal meningitis. That’s a lot easier to say than pneumonia caused by Streptococcus pneumoniae, or meningitis caused by Neisseria meningitidis.
Some diseases have rather expansive names like acquired immune deficiency syndrome or severe acute respiratory syndrome. It’s much easier to simply reduce it to a few letters, such as AIDS or SARS. Occasionally it is the organism’s name that becomes reduced. Methicillin-resistant Staphylococcus aureus becomes MRSA and extended spectrum beta-lactamase producing Enterobacteriaceae becomes ESBL.
The name of the place where a disease or its causative organism was first described was, at one time, a convenient way to assign a name. Rocky Mountain spotted fever, Legionnaire’s disease (described after its discovery at a convention of the American Legion at a Philadelphia hotel in 1976), Norwalk agent (now Norovirus), and Ebola (a river in Africa) are examples. This usually occurred when the causative organism and the disease course wasn’t well established.
One of the more well-known infectious diseases named for the place of discovery is Lyme Disease, first described in the communities of Lyme and Old Lyme, Connecticut. This is a convenient name as it is simple to say, but it is most unjust for the good folks residing in the beautiful areas in southeastern Connecticut. (There are actually three communities, Lyme, Old Lyme, and East Lyme. The disease was first described in the area of the first two). The disease certainly did not originate there; it is found in many parts of the world. It was the perspicacity of two mothers, a local physician, and some state public health officials that brought to light the nature of the disease and its cause. The workers in Lyme, Connecticut, in the 1970s deserve many accolades for describing the disease, not the infamy of its name.
In 2015 the World Health Organization issued guidelines for naming newly discovered diseases, eliminating the use of geographic locations. But for those already established, the name usually persists.
There are three essential participants in the cycle of Lyme disease: (1) ticks of the genus Ixodes, (2) the causative organism Borrelia burgdorferi, and (3) the host animal. From the organism’s perspective, the much-preferred animal host is the white-footed mouse or a chipmunk. It can infect the rodent, make many copies of itself in its tissues, and end up in the bloodstream. When another tick comes along and takes a blood meal, the organism can then be passed onto another animal host, perpetuating the bacterial species. While the rodent is infected, it is not sickened. The bacteria and the small creature get along quite well, with no ill effects to either. The bug has just enough control over the little animal’s immune system to allow it to replicate without damaging the host. A “good” parasite does not want to damage the host. It needs the animal to stay relatively healthy so it can escape and get on to the next one. The relationship between Borrelia burgdorferi and the white-footed mouse and chipmunks is just such a compatible arrangement.
Trouble arises when the organism makes its way into a host, like a human, with an immune system that is in some ways different than the primary host. In this case the host (the human) is sickened. The bacterium has enough control over the host’s immune system to allow itself to persist, but at the expense of creating numerous detrimental immune reactions. The organism does not reach high enough numbers in the bloodstream to enable it to be sucked up by a second biting tick, and humans don’t get that many tick bites. For the organism, infecting a human is a dead end.
Ticks can bite some animals but not spread the disease. An important mammal in the chain is the white-tailed deer. While not seriously sickened by the bacterium, deer are large animals that many ticks can bite. They also can roam over wide geographic locations, taking their tick passengers with them. Those ticks can then mate, produce eggs in new areas, and begin a cycle of infection with nearby rodents, which can spread the bacteria to other ticks. When deer populations increase, so do tick populations, broadening the possibilities for disease expansion.
There are two types of ticks, hard and soft. The obvious difference is a solid plate, called a scutum, covering the back of the former. They live in different places, with hard ticks dwelling in open fields on grasses and brush, while soft ticks usually live in enclosed areas like animal burrows. Both can spread disease, but the hard ticks are responsible for most of them.
The name tick comes from several European languages, meaning “touch.” The word tickle has the same roots. Ticks are eight-legged and are classified with spiders in the class Arachnida. There are 14 genera of hard body ticks, but the ones responsible for spreading infectious diseases to humans are members of the genus Ixodes. The name Ixodes is from the Greek word for “bird-lime,” a sticky substance applied to tree branches to trap birds, much like fly-paper traps flies.
There are many species of Ixodes, but only a few are associated with Lyme disease, and it varies with location. In the eastern U.S. it is Ixodes scapularis; western U.S., Ixodes pacificus; in Europe, Ixodes ricinus; in China, most likely it is Ixodes persulcatus. Environmental factors play a major role in the occurrence of Lyme disease, as there are many parts of the story, not the least of which is the number of mice and other suitable animals in a locality. Sometimes reforestation, returning farmland to a natural setting, enhances the population of mice, deer, and other animals in an area. If humans frequent this area for recreation or work, and ticks are present, the disease frequency increases. This is most likely what happened in Old Lyme, Connecticut, when the disease was first described.
Ticks go through three distinct stages after hatching from eggs: larva, nymph, and adult. We can think of it in people terms as toddlers, teenagers, and adults. Each stage requires blood from an animal for its survival. Unlike mosquitoes, where only the females take blood meals, both male and female ticks feed on blood. The female adults take a whole lot more since they make eggs and need to nourish them.
Ticks don’t fly like mosquitoes, so they have another method of finding a suitable host. After hatching, larvae crawl to the end of a blade of grass or piece of shrubbery. With their hind legs they hang onto the vegetation. Their front legs have little barbs on them, and they sit there all day waving them around, waiting for an animal to come by. They are on a quest to find a suitable animal to suck its blood, and the process has the appropriate name “questing.” When a little animal wanders by, they “grab” onto the unsuspecting animal and hop aboard. Then they crawl over the host’s body, looking for just the right spot to attach. With humans that would be soft folds in the skin. The armpits and neck are prime locations, but there are several others. Nymphs, larvae, and adults all follow the same pattern.
Mosquitoes are in and out quickly. They land, insert their needle-like proboscis, take their blood, then fly away, the whole process taking just a few seconds. Ticks, on the other hand, need several days to get their blood meal, so they have evolved special means to allow that to happen. They must stay attached, so a firm grip is essential. The mouthparts by which they attach have a barb on them that allows them to stick. They must operate surreptitiously so the host animal won’t try to remove them, and to that end they have two elegant properties. For one, they insert an anesthetic into the bite, making it painless. They also have the means to inhibit the local immune response to their presence. Think about getting a thorn in your finger; what happens after a day or two? Red, swollen, and painful. The same thing would happen around the attached tick, but they can circumvent the inflammatory response to their presence. Unfortunately for us, that immune mitigation works in favor of any pathogenic organisms they are carrying. Ticks also must ensure that the blood they are removing doesn’t clot, so an anti-coagulant is a must.
Ixodes feeds on blood at all three stages, larva, nymph, and adult. It doesn’t matter to them what kind of blood they obtain; bird blood is just as good as mammal blood. Some species also feed on reptiles. The Lyme disease-causing bacterium they may carry, Borrelia burgdorferi, does have a preference, and it is the mammalian species in which they can propagate and move about with impunity. Field mice and chipmunks fill the bill. In other species they can enter and set up an infection, but usually they meet their end if in the wrong host. Of course, they are merely passengers and go where their tick host takes them.
Borrelia burgdorferi does not survive over winter in Ixodes eggs, so the larvae are not infected and cannot inject the bacteria when they take their blood meal. If the blood of the animal they are feeding on is infected, they will pick up a load of bacteria with their blood. The larva usually doesn’t need to feed again that season, but the bacteria have a way of staying alive in the young tick, even when it molts and becomes a nymph. When the nymph feeds, the bacteria it is harboring can then infect the new animal host. The same applies when the nymphs molt into adults.


The causative agent of Lyme disease is Borrelia burgdorferi. The genus is named for Amadee Borrel, a French microbiologist who worked to distinguish the groups of spirochetes. The species is named for Willy Burgdorfer who discovered the Lyme disease bacterium in 1982.
Borrelia burgdorferi differs significantly from other pathogenic bacteria. The most obvious distinguishing characteristic is their spiral, corkscrew-like shape. Flagella are little hairs that project from bacteria and beat rapidly to propel the organism through its environment. Borrelia has flagella, but they are internal, running up the inside of the bug from one end to the other. This is where they get the power to move so strongly in their corkscrew fashion.
The cell walls of Borrelia are like Gran-negative organisms, but with one great exception: they don’t have lipo-polysaccharide, commonly known as endotoxin, which is present in nearly all Gram-negative bacteria. Instead, they have a wide array of different lipoproteins in their cell wall. This makes them unusual among bacteria, and the immune system must adapt over time to recognize these many differing lipoprotein antigens.
Another feature of their cell wall is that they don’t have the enzymes to recycle the wall’s main component, peptidoglycan. Rather than being conserved and incorporated into other growing bacteria, Borrelia peptidoglycan flies off in every direction. This is significant in disease as the immune system is primed to detect and react to peptidoglycan as a foreign invader, whether it is attached to an organism or not.
Of particular note in Borrelia burgdorferi is its genetic makeup. Like all bacteria, it has one long strand of DNA that codes for its necessary structures and metabolism. The genes on this strand are highly conserved, and one strain of the organism looks just like another. But the organism can accumulate many smaller plasmids, usually round strands of DNA independent from the main chromosome. Over 20 different plasmids have been detected in Borrelia burgdorferi. In contrast to the main chromosome the plasmids are highly variable, both in their presence or absence within the organism and in their content. As a result, there are myriad strains of the organism in nature. Which one ends up in a human patient is a matter of chance.
Borrelia burgdorferi is unique among pathogenic bacteria because it does not produce toxins or penetrate host cells. It causes damage through its genetic variability and the immune system’s response to it.
For Borrelia burgdorferi the animals it finds itself in differ radically, one being an insect and the other a mammal. Accommodations must be made. The organism is not free living, so it can’t use its own enzymes to generate food. It must obtain all its nutrients from the animal in which it resides, whether insect, mammal, bird, or reptile.
When residing in its tick reservoir, the organism shuts down its own metabolism. It expresses a protein on its outer coat, allowing it to adhere to the tick's midgut. It can’t do much else but just sit there, almost like it’s in hibernation. When the tick feeds, though, everything changes. The blood that comes rushing in sends a signal to the organism’s genome to shut down the protein production that allows it to stick to the wall of the tick’s tummy and make its way to the tick’s salivary glands. They then begin producing the proteins that will allow it to proliferate in its new mammalian host. First among these are the internal flagella, and the substances that will enable it to avoid the attack of complement.
Once inside the new mammalian host, the tick’s invasive properties greatly aid the bacteria. There is the absence of pain, anti-coagulation, and the reduction of a localized inflammatory response, primarily neutrophils and complement. After entering the skin, the bacteria begin proliferating and moving every which way with their very active flagella. With their spiral shape and powerful flagella, they are no match for the cumbersome neutrophils and macrophages trying to capture them, and they disseminate quite far. They also produce a substance that acts very much like interleukin-10, an anti-inflammatory cytokine that slows down neutrophil induction.
The immune system catches up with them within a few days, and inflammation develops. The tell-tale sign is usually a distinctive rash described as a “bullseye,” with a solid inner round red area, immediately surrounded by normal colored skin, then another round red circle. It is technically called erythema migrans, with erythema meaning redness and migrans meaning moving. They can be anywhere from a few inches to a foot around. It’s all rather bizarre, really, but nonetheless distinctive. Sometimes the rash is not in the traditional bullseye shape but just a rash. It forms due to the innate immune system’s effort to capture and kill the invading bacteria. Antibiotic treatment is usually very effective when applied at this first sign of trouble.
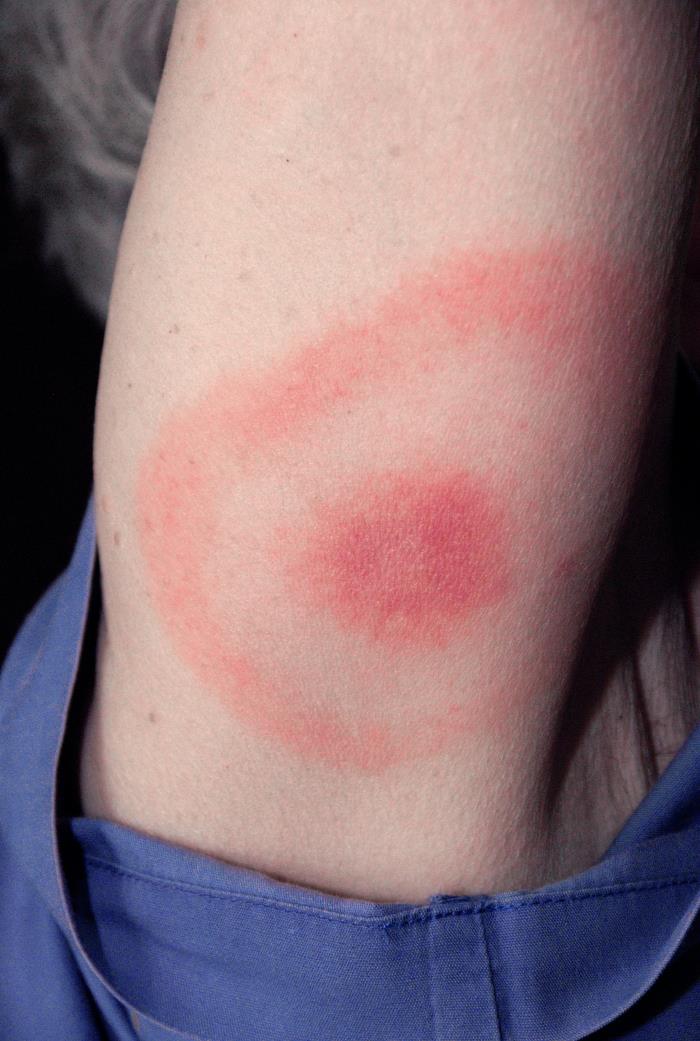
If only it were that simple: bullseye rash, antibiotics, end of story. Unfortunately, life sometimes throws us curveballs. Some people don’t develop the distinctive rash; if they do, it is on their back where they can’t see it. Some get the normal course of antibiotics and go on to develop significant symptoms anyway. A lot depends on the individual involved and the strain of bacteria. There are many variations.
Because of its robust motility, Borrelia burgdorferi can easily make its way into the blood vessels and lymph channels, allowing it to spread to every area of the body. The bugs can theoretically go anywhere, but they have a particular fondness for joints, the heart, and the central nervous system. They don’t produce toxins and they don’t penetrate cells. Instead, they firmly attach themselves to protein components of the extracellular matrix. That’s the complex of tough fibers outside cells that support tissues and allow cells to communicate. The chief proteins of this matrix are decorin (a small protein associated with collagen), integrins, fibronectin, and others. Borrelia burgdorferi has proteins that allow for firm attachment to each one of these.
One would think that a bacterium just sitting outside a cell and attached to a protein would be a sitting duck for the immune system. But the organism has the means of allowing itself to survive. For one, it contains a protein known as CD47, commonly known as the “Don’t eat me” protein. It is a protein expressed on human cells when they need to survive and cannot have macrophages eliminate them. Borrelia burgdorferi expresses the same one, avoiding macrophage engulfment.
Complement is the great enemy of bacteria, either acting alone or in concert with neutrophils. Borrelia is very good at circumventing the activity of complement, both early and late in the infection. An important regulator of the active C3b portion of complement is Factor H. The organism contains at least five proteins that bind Factor H, which then binds to the C3b molecule inactivating it. The effectiveness of complement is greatly lessened.
Disguise is another weapon the organism is very good at. All invading microorganisms have on their surface substances, either protein or carbohydrate, that are unique to them and foreign to the host animal. Known as PAMPs, or pathogen associated molecular patterns, they are prime targets for the cells of the immune system. Building antibody and T-cell responses to these microbial surface substances allows the host, through its adaptive immune response, to quickly rid itself of the pathogen.
The most common PAMP for Gram-negative bacteria is lipo-polysaccharide, or LPS. It is highly antigenic as several human proteins can detect its presence and signal a robust immune response. The problem with Borrelia burgdorferi is that it doesn’t contain LPS, and the usual detection means by the immune system are rendered useless. But it does have nearly a hundred different alternative lipoproteins in its cell wall. These cell wall lipoproteins stimulate the innate immune system to produce cytokines, especially tumor necrosis factor and interleukins 6 and 8. Interferon is also produced. The abundant production of these cytokines creates most Lyme disease symptoms.
The deceptive technique the organism has mastered is the routine switching of the lipo-proteins in its cell wall. One day one lipoprotein is present, a few days later another, and on and on as the bug is adept at changing them out. Antibody and T-cell response to one is useless with the subsequent iterations, allowing the organism to escape the ravages of the adaptive immune system. The main reason for this protein switching ability resides in a bacterial protein known as Vls-E. Its genes reside near the end of a plasmid designated Pp28-1. Located nearby the Vls-E gene are 15 other genes that code for similar but notably different lipoproteins. This arrangement allows the organism to mix and match genetic material, giving many combinations and resulting in lipoprotein differences.
These well-developed characteristics of rapid motility, complement avoidance, “don’t eat me” protein, adhesin to the extra-cellular matrix, and ever-changing surface lipoproteins make for a very persistent bacterium. In nature, infected mice never get rid of it. Once infected, always infected. Fortunately for the mice, the organism doesn’t do them any great harm. They live in a relatively benign co-existence. Unfortunately, that doesn’t apply to human’s relationship with the spirochete. Our immune response is sufficiently different from that of mice, so many infected people have severe symptoms. The symptoms in humans are not the result of direct injury by the organism; they produce no toxins and do not enter cells. But they persist for long periods of time, and the activity of our immune system in trying to get rid of them causes the symptoms.
One pernicious protein of the Lyme bacterium is a cell wall lipoprotein known as Arp. That stands for “arthritis related protein.” The organism can infect a great many tissues, but it has a predilection for joints. Perhaps those tissues offer more receptors for its attachment proteins, or it can hide better there. Whatever the reason, arthritis is a hallmark of the disease, with about 60% of infected persons displaying symptoms. Arp is an essential part of arthritis pathology, as tests in mice with organisms lacking Arp don’t develop joint inflammation. Just what Arp does is not known, but it seems that it acts as a signal to the immune system to produce an abundance of inflammatory substances and thus induce joint inflammation and pain. It also seems that various forms of VlsE, the cell wall lipoprotein, act as a shield to protect Arp from an immune response.
Lyme disease was unknown in the 1970s, but it is currently recognized as the most common insect-borne disease in the United States, with over 300,000 cases annually. It is a very complex organism and disease progression with many novel and unpredictable facets. Borrelia burgdorferi’s ability to elude and modulate the immune system, its many different surface proteins, its capacity to invade many different tissues of the body, and its potential to shut down much of its activities and exist in a near-hibernating state make for a disease with many presentations and outcomes. Lyme Disease can be one of the easiest infectious diseases to diagnose, and it can also be one of the most challenging and exasperating.
A few simple facts can lead to a quick, accurate diagnosis: history of a tick bite in an area where the disease is known to be endemic, and the tell-tale rash appearing a few weeks afterward. If the patient sees a physician at this stage, is prescribed an appropriate antibiotic, and complies with taking it for the prescribed duration, most Lyme Disease cases are cured. That’s most, not all. A few people develop chronic symptoms despite compliance with appropriate early therapy.
Where things can get complicated is when early diagnosis is missed. The spirochetes will end up in many different parts of the body, and many different symptoms can be displayed. With many bacterial infections the isolation of the causative organism confirms the diagnosis. Appropriate samples from the patient are sent to the clinical laboratory, and direct observation under the microscope or bacterial culture, or both, are enough to confirm the diagnosis. This can’t be done with borreliosis. The organism is too small in number in the blood to be seen under the microscope and does not grow in routine bacterial culture. Also, it hides out in obscure locations throughout the body, so getting an appropriate sample is not possible.
Lyme disease diagnosis depends entirely on looking for antibodies our body produces to the organism. This is complicated, especially for a resourceful organism like Borrelia burgdorferi. The bug is notorious for changing its surface material, so the antibodies produced can be non-specific. Other unrelated organisms may have just happened to produce a similar antigen, so the antibody to it may appear just like the one made to Borrelia, confusing the diagnosis. Lyme disease symptoms frequently mimic those of other diseases, so if the patient does not relate a travel history that would tip off a physician as to a possible infection, other avenues of illness and their causes may be explored needlessly.
The most common symptom of Lyme Disease other than the rash is arthritis. It was the unusual occurrence of arthritis among children in Connecticut in the 1970s that prompted researchers at Yale University to pursue investigations into the then-unknown disease. The knee is most commonly affected, but it can also involve other joints. Typically, there is redness, swelling, and pain. It is not the live organism burrowing through the joint that causes the symptoms, but the immune system’s reaction to the organism, or, more likely, parts of the organism, that produce the reaction. As noted, Borrelia burgdorferi does not recycle the components of its cell wall known as peptidoglycan. It all is cast off like so much microbial rubbish. Peptidoglycan is immunogenic; the immune system reacts to it, and if it is concentrated in joint tissue, there will be an immune reaction that results in swelling and pain. The treatment of choice is often cortico-steroids to tamp down the immune response.
Another symptom of Lyme disease that appears in some patients involves the heart. Lyme carditis, when it occurs, happens quite early in the disease, just a few weeks after the tick bite. The bacterium likes to settle on extra-cellular matrix proteins, with the protein decorin being one of its favorite targets. Heart tissue contains a lot of decorin, and in some, but not all, patients, there is a notable adhesion of the organism to this protein. The result is a disruption of the electrical signals from the heart's upper chambers to the lower, often referred to as a heart block. Patients experience palpitations, shortness of breath, light-headedness, and sometimes chest pain. A few experience cardiac arrest.
Lyme carditis is not very common in Lyme disease patients, perhaps one in a hundred. The reason is unclear, but there may be a genetic difference in the structure of the decorin in these individuals, and the organism attaches more strongly. Without a known history of tick exposure, the diagnosis can be most troublesome, as it occurs early in the progression of the disease when antibody formation is not complete, and the serology studies can be misleading.
Lyme disease can also affect the central nervous system, giving symptoms such as numbness, double vision, and facial palsy. Sometimes more severe symptoms such as meningitis or encephalitis occur. The organism doesn’t penetrate and navigate through human cells, but it is very good at traversing the junctions between them. They can thus penetrate the blood-brain barrier, and trouble ensues.
In the United States, most cases of Lyme disease go undetected and unreported. The Centers for Disease Control gets reports of about 30,000 cases annually, but they feel there are probably ten times that many cases. Early detection and treatment are key; most cases disappear after a couple of weeks of antibiotics such as doxycycline or amoxicillin. But as shown, Lyme disease can be the great imitator, with symptoms resembling those of other ailments and confusing diagnoses. A significant percentage of patients, probably around 10-15%, suffer long-term consequences of the disease. Just why is subject to speculation, but some reasons may be the organism’s ability to hide itself and change its surface proteins, the mass production of bacterial by-products such as the cell wall component peptidoglycan that are immunogenic, the bacterium’s ability to enter a quiet, non-metabolic state rendering it tolerant to antibiotics, the great variation in the different strains of organisms that can infect humans, and differences in the genetic make-up of the human hosts. These are not mutually exclusive, so several can apply in a single case.
Photos are from the Public Health Image Library, CDC
